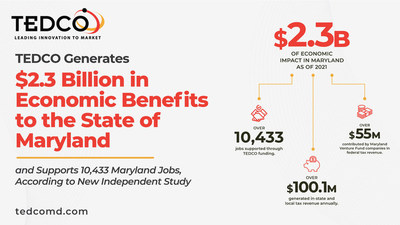
Funding for the technology infrastructure must go to a nonprofit, such as a university, but the nonprofit will need the commercial real estate industry to design, build and manage this technology infrastructure.
The District of Columbia/Maryland/Virginia (DMV) region submitted some very good bids, including ones in quantum technology, biotechnology, advanced manufacturing, and other topics but only Howard County for cyber security, Richmond, Virginia in bio manufacturing, and rural Southwest Virginia in transportation logistics won planning grants.
Given the region’s enormous amount of research and development, universities, federal labs, HBCUs, and other assets, it might have been expected that the three-jurisdiction region would have had more winning Phase 1 proposals. The District of Columbia did not win a grant at all.
One thing hampering the region’s efforts to win EDA funding is a lack of a regional CEDS — Comprehensive Economic Development Strategy. A CEDS is a necessary component of any EDA grant submission. Many counties in the DMV do not have a formally approved CEDS. Previously, CEDS were not that important, as the EDA had relatively little funding and much of it was restricted to economically distressed areas for water and sewer projects.
However, all of that has changed as the EDA has declared the entire U.S. as a distressed region, and Congress has given EDA an enormous amount of funds for a variety of purposes, including billions for new regional technology infrastructure grants.
The U.S. Innovation and Competition Act currently under consideration by Congress authorizes the EDA to receive approximately an additional $10 billion in future years for projects, including regional technology hubs. So, while the region might have punched under its weight with this round, our region and the real estate industry need to be prepared for the next.
Luckily, D.C., Maryland and Virginia were each given $1 million in planning funds by the EDA to do individual economic development strategies. Some of these funds could be used for the regional overlay purpose. The region should learn from the states of Massachusetts, Rhode Island, and Maine that collectively won an EDA planning grant for regional biotechnology manufacturing.
Adding a DMV regional CEDS overlay strategy in areas such as quantum, biotechnology, AI/machine learning, advanced manufacturing, aero and space tech, minority and small business development, HBCU outreach, community college involvement, bio manufacturing, workforce development, federal lab partnerships and other topics would help the region and real estate industry.
The Greater D.C. Partnership and Connected DMV have been doing great work, and adding a regional CEDS overlay zone is a practical way to increase odds of winning EDA funding in the future and creating greater regional cohesion, like our peers across the country.
Brian Darmody
CEO
Association of University Research Parks (AURP)
AURP DC Area Office
7761 Diamondback Drive
College Park, MD 20742
301-928-0527
www.aurp.net